Vero Cell Vaccine Covid-19 Effectiveness / Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
The available data on the Sinovac-CoronaVac COVID-19 vaccine in pregnant women are insufficient to assess either vaccine efficacy or possible vaccine-associated risks in pregnancy. So far Vero is the only Chinese vaccine for which the manufacturer has published official data.

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News
COVID-19 Vaccine Vero Cell Inactivated also contains an adjuvant a substance that helps strengthen the immune response to the vaccine.
Vero cell vaccine covid-19 effectiveness. Comparison of the proportions of confirmed Covid-19 cases in the two vaccine groups and the placebo group. Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases Total Subject Number Person year. China joined this club of elites of science following the Chinese FDA approval of Sinopharm the subsidiary of state-owned China National Pharmaceutical Group- CNPG first COVID-19 vaccine inactivated Sars-Cov-2 based on the results of the phase-3 clinical trial in UAE and Bahrain showing up to 86 efficacy of the vaccine in preventing COVID-19.
Doses of the Chinese Sinopharm vaccine against the coronavirus disease COVID-19 are seen at the Biblioteka kod Milutina restaurant in Kragujevac Serbia May 4 2021. However this vaccine is an inactivated vaccine with an adjuvant that is commonly used in many other vaccines with a well-documented safety profile such as. Individuals with a history of anaphylaxis to any component of the vaccine.
Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac. When a person is given the vaccine their immune system identifies the inactivated virus as foreign and makes antibodies against it. COVID-19 vaccine efficacy from clinical trials COVID-19 vaccines licensed for use in the EUEEA have been shown during clinical trials to be highly effective in providing protection against symptomatic COVID-19 and severe disease.
Vero cell vaccine effectiveness. COVID-19 vaccine effectiveness - real-world evidence. Providing Covid-19 vaccines to outgoing migrants was necessary.
All confirmed cases are all confirmed by DSMB blind examination. When medicines are under rolling review EMAs. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
Cells used for vaccine manufacture are the working Vero cell bank which is of the 142nd generation. EMA will assess the compliance of COVID-19 Vaccine Vero Cell Inactivated with the usual EU standards for effectiveness safety and quality. Nepal is set to start the second phase of its COVID-19 inoculation drive using China-made Vero Cell vaccine sans concerns over safety and approval from the.
Kathmandu Nepal April 4 ANI. Once inactivated viruses get presented to the bodys immune system they stimulate the production of antibodies and make the body ready. Nepal has granted emergency approval to the chinese vero cell vaccine given the active caseload that stands at 277944 on monday while 273240 people have recovered in nepal.
Who is the vaccine not recommended for. Protective effect against COVID 19 after 14 days following the full course of vaccination among healthy population aged 18 years old and above. The SARS-CoV-2 Vaccine VeroCell is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys immune system without risk of causing disease.
Vaccine efficacy against laboratory-confirmed symptomatic COVID-19 was estimated to be 78 in adults 18-59 years of age. The addition of this vaccine has the potential to rapidly accelerate COVID-19 vaccine access for countries seeking to protect health workers and populations at. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell.
Doubts Over China Vaccines Effectiveness Mar. The manufacturer has not released official figures on the efficacy of the CoronaVac vaccine but evaluations by the Brazilian collaborators suggest an efficacy of up to 78. Detail of trials of Sinopharm inactivated COVID-19 vaccines Vero Cells.
Sinopharm vero cell vaccine efficacy. While EMA cannot predict the overall timelines it should take less time than normal to evaluate an eventual application because of the work done during the rolling review. Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac.
The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements. The Covid-19 vaccine made by Chinas Sinopharm was less effective than others at preventing infection hospitalization and death especially among people over 50 according to a. Vero cell vaccine sinopharm or sinovac.
The governments decision to administer the Vero Cell vaccine developed by Chinas Sinopharm is not the right choice of vaccine for migrant workers stakeholders say. It is the also first vaccine that will carry a. If anything the vaccine choice has added to the complication.
Table 1 shows the overall vaccine efficacy of the licensed vaccines after completed vaccination. The effectiveness of the Covid-19 vaccine BIBP in pregnant women is therefore expected to be comparable to that observed in non-pregnant women of similar age. But now there is a new problem said Sujit.
On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation.

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine
Singapore Excludes Sinovac Shots From Covid 19 Vaccination Tally Nikkei Asia
Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
China S Vaccine Diplomacy Assumes Geopolitical Importance Merics

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
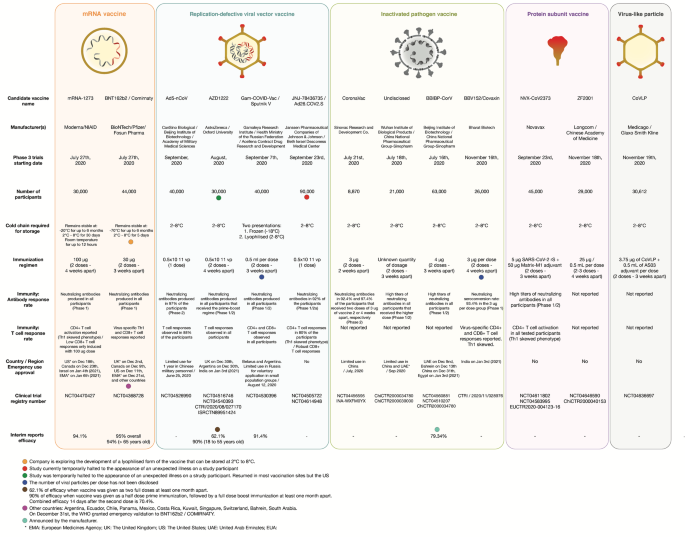
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
Doubts Over China Vaccines Effectiveness Mar Production Push Nikkei Asia

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
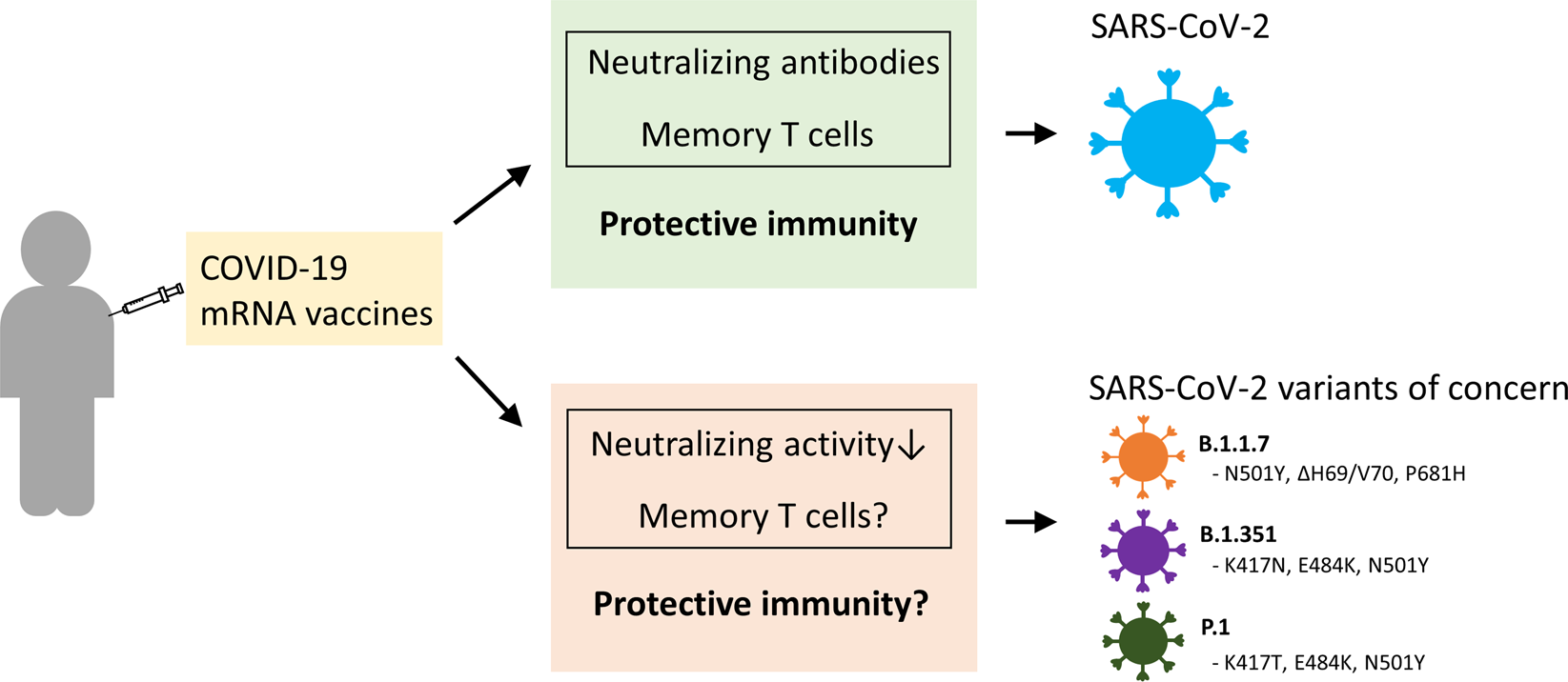
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
Expert Chinese Vaccines Effective Against Covid 19 Delta Variant Cgtn

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld

.JPG)
