Vero Cell Covid Vaccine Efficacy Rate / Sinopharm S Two Covid 19 Shots Effective Study Says Reuters
COVID-19 Vaccine Janssen 669 95 CI 5903 7340 18 years against COVID-19 of any severity 767 95 CI 546 to 891 against severe-critical COVID-19 5 21 September 202022 January 2021. COVID-19 Vaccine Vero Cell Inactivated detailed edition Created Date.

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Interval of 21 days had the efficacy of 79 against symptomatic SARS-CoV-2 infection 14 days or more after the second dose.
Vero cell covid vaccine efficacy rate. Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases Total Subject Number Person year. For all vaccines we assume 75. The companys update came a few days after the National Institute for Allergy and Infectious Diseases NIAID expressed concern over data AstraZeneca had submitted in advance of requesting an EUA from the FDA.
To assess either vaccine efficacy or vaccine. The efficacy rating followed an interim report of ongoing human trials conducted in that country CNBC reported. Estimated Primary Completion Date.
They provided no details on clinical testing. Purified Vero cell rabies vaccine is safe carries a very low adverse reaction rate and is effective in preventing rabies in severely exposed subjects when used with human or equine rabies. 95 cases in placebo group.
Public health authorities across the globe have been mobilising to deliver the biggest ever vaccination programme to battle Covid-19. The Covid-19 vaccine BIBP in pregnant women is. The trial includes two parts namely the efficacy study and immunogenicity bridging study.
On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation. For other mRNA vaccines we assume the same efficacy as Pfizer-BioNTech. This is a randomized double-blinded placebo controlled phase III clinical trial to evaluate the efficacy safety and immunogenicity of SARS-CoV-2 Vaccine Inactivated Vero Cell in adults aged 18 years and above after 2-dose schedule.
So far Vero is the only Chinese vaccine for which the manufacturer has published official data. The company also said the vaccine was 85 effective in preventing COVID-19 in people over 65. 10 11 Peer-reviewed results published in JAMA of Phase III trials in United Arab Emirates and Bahrain showed BBIBP-CorV 781 effective against symptomatic cases and 100 against severe cases 21 cases in vaccinated group vs.
SARS-CoV-2 exhibits extremely efficient human-to-human transmission the new pathogen rapidly spread worldwide and within months it caused a global pandemic changing daily life for billions of people. We also used the vaccine effectiveness against severe disease for the Johnson Johnson vaccine instead of the efficacy against all symptomatic disease using results from the clinical trial. The median duration of follow up available at the time of review was 112 days.
It had also recently announced that another of its COVID-19 vaccines Soberana 2 had an efficacy of 62 after two of its three shots. How Does COVID-19 Vaccine Covaxin Work. The vaccine was.
How many people have received the Covid-19 vaccine. 704 958 548-806 overall vaccine efficacy against COVID-19 across both dosing groups 4 23 April 20204 November 2020. COVID-19 Vaccine Vero Cell Inactivated detailed edition Title.
A large phase 3 trial in Brazil showed that two doses administered at an interval of 14 days had an efficacy of 51 against symptomatic SARS-CoV-2 infection 100 against severe COVID-19 and 100 against hospitalization starting 14 days after receiving the second dose. We bring you the latest Covid-19 vaccine numbers including daily Covid-19 vaccine rates per country. A total of 34020.
Vaccine efficacy against hospitalization was 79. The COVID-19 vaccine Covaxin has shown an efficacy rate of 78 which is pretty good considering the circumstances and is 8 more than Serum Institute of Indias Covishield the other vaccine being available in the country. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell.
Actual Study Start Date. The Cuban government announced on June 22 2021 that its three-shot Abdala vaccine has a 92 efficacy rate against COVID-19. Secondary efficacy endpoints are the incidence of hospitalizationmortality rates among one or two dose regimens duration of immunogenicity rates up to 120 days the seroconversion rate the seropositivity rate neutralizing antibody titer and IgG levels 14 days after each dose of vaccination.
The COVID-19 case fatality rate of 25 makes. Pfizer and BioNTechs Covid-19 vaccine is just 39 effective against delta in Israel but still provides strong protection against severe illness and hospitalization. The trial was not designed and powered to demonstrate efficacy against severe disease.
Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac. Multicenter Randomized Double Blind Parallel Placebo Controlled Phase III Clinical Trial to Evaluate the Protective Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above. The primary efficacy endpoint is the incidence of symptomatic cases of COVID-19 disease confirmed by RT-PCR two weeks after the second dose of vaccination.
The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning. Coronavirus vaccine roll out statistics by country.
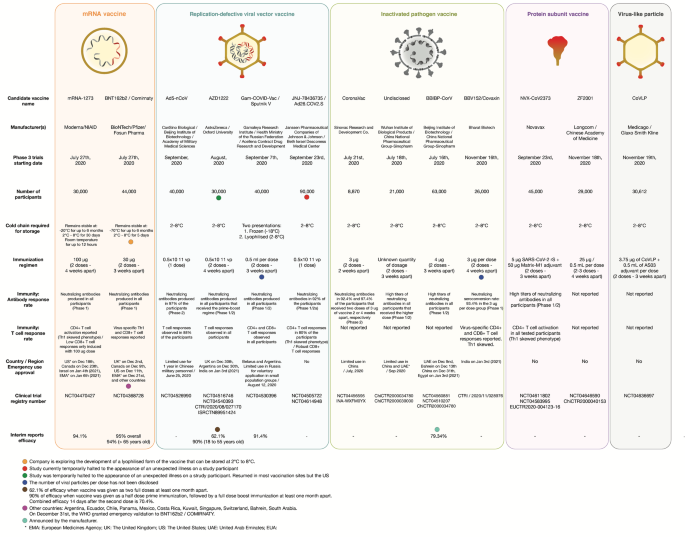
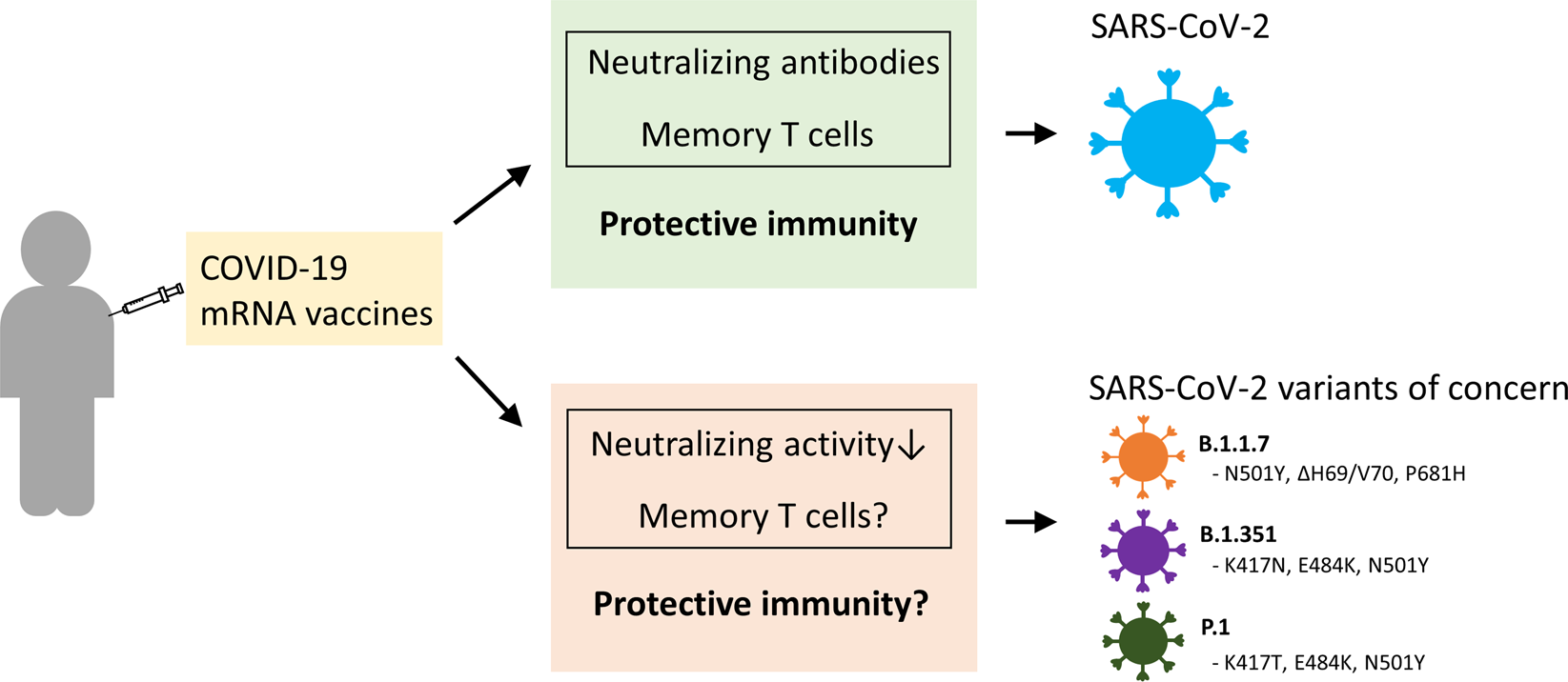
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
Covid Bolsonaro Hails Suspension Of Chinese Vaccine Trial Bbc News
Peru Study Finds Sinopharm Covid Vaccine 50 4 Effective Against Infections Dhaka Tribune
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace

China Grants First Approval Of Homegrown Covid 19 Vaccine Voice Of America English

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December

Integrated Control Of Covid 19 In Resource Poor Countries International Journal Of Infectious Diseases

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters
Top Chinese Official Admits Vaccines Have Low Effectiveness Abc News

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
